Thousands of lawsuits have been filed over the past six years against various manufacturers of surgical mesh products used to treat conditions such as pelvic organ prolapse and stress urinary incontinence in women. These lawsuits challenged the safety and effectiveness of these products, after years of reports of women experiencing pain, bleeding, infection and other complications caused by the mesh implants. Often, the complications resulted in women having to undergo multiple surgeries to repair or remove the mesh product. Now, the United States Food & Drug Administration (FDA) is finally getting on board with those of us who have been stating for years that these products are neither safe nor effective.
In July of 2011, the FDA concluded that women who were surgically implanted with vaginal mesh products had more complications than those who underwent traditional surgery involving stitches. At that time, the FDA issued an update on serious complications associated with the transvaginal placement of mesh for the condition known as pelvic organ prolapse (POP). This condition occurs when the tissue that holds the pelvic organs in place becomes weakened or stretched, resulting in the organs prolapse or bulge into the vaginal area, causing various and serious complications. In its 2011 update, the FDA informed doctors and patients that serious complications associated with the use of transvaginal mesh products for repair of pelvic organ prolapse were “not rare.”
This week, the FDA took a stronger stand against the use of surgical mesh products to repair POP in women, classifying transvaginal mesh products as “high-risk” medical products and subjecting them to additional regulatory scrutiny. Previously, these products were considered “moderate-risk” products. Now, the manufacturers of pelvic mesh products will have to submit new applications to the FDA, demonstrating both the safety and effectiveness of these mesh devices.
 Georgia Injury Lawyer Blog
Georgia Injury Lawyer Blog



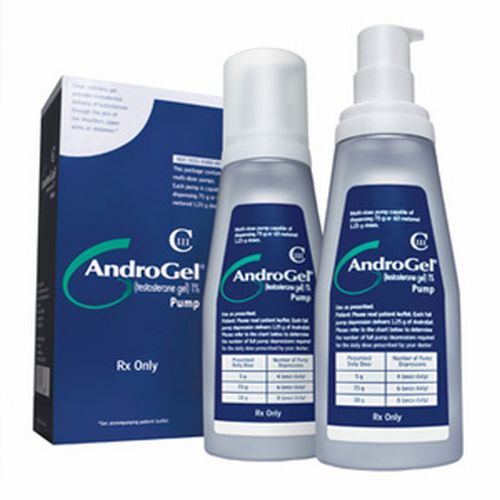 AndroGel testosterone gel is a topical testosterone replacement therapy (TRT) manufactured by AbbVie, Inc. and formerly Unimed Pharmaceuticals of Abbott Laboratories, Inc. AndroGel is approved by the Food and Drug Administration (FDA) to treat men with low testosterone or “Low T,” associated with a diagnosed medical condition. AndroGel 1% and AndroGel 1.62%, are both applied topically to the skin. Patients apply it directly to their upper arm and shoulder and it is absorbed through the skin to deliver testosterone to the patient for approximately 24 hours. The drug has been heavily prescribed over the past several years as a safe way to treat men with low testosterone. However, numerous recent studies suggest that men taking AndroGel have a far greater risk of suffering a heart attack, stroke, congestive heart failure, or other adverse cardiovascular problem. The most recent study, known as the PLOS ONE study, performed on January 29, 2014, found that men 65 years and older who took testosterone injections or used the gel, had double the risk of a heart attack in the months after starting the treatment. On Jan. 31, 2014, after reviewing these studies, the FDA announced it would be investigating the risks of heart attack, stroke and death in men using prescription testosterone products.
AndroGel testosterone gel is a topical testosterone replacement therapy (TRT) manufactured by AbbVie, Inc. and formerly Unimed Pharmaceuticals of Abbott Laboratories, Inc. AndroGel is approved by the Food and Drug Administration (FDA) to treat men with low testosterone or “Low T,” associated with a diagnosed medical condition. AndroGel 1% and AndroGel 1.62%, are both applied topically to the skin. Patients apply it directly to their upper arm and shoulder and it is absorbed through the skin to deliver testosterone to the patient for approximately 24 hours. The drug has been heavily prescribed over the past several years as a safe way to treat men with low testosterone. However, numerous recent studies suggest that men taking AndroGel have a far greater risk of suffering a heart attack, stroke, congestive heart failure, or other adverse cardiovascular problem. The most recent study, known as the PLOS ONE study, performed on January 29, 2014, found that men 65 years and older who took testosterone injections or used the gel, had double the risk of a heart attack in the months after starting the treatment. On Jan. 31, 2014, after reviewing these studies, the FDA announced it would be investigating the risks of heart attack, stroke and death in men using prescription testosterone products.